As plants interact with the environment directly by exchanging water and energy, they are very sensitive to storms, droughts and floods.
As plants interact with the environment directly by exchanging water and energy, they are very sensitive to storms, droughts and floods. The Role of Plants in the Environment
Plants are necessary for all life on Earth. Plants provide many things for the sustainability of life on our planet.
As a critical part of the ecosystem, plants provide oxygen for organisms to survive. They are able to reduce the problem of pollution, by using carbon dioxide. Plants are also the basis of most food webs as producers of food for herbivores and ultimately carnivores. Plants also provide shelter for animals, clean and filter water and help prevent soil erosion.
This picture shows some of the more minor ways in which plants interact with their environment. Environmental conditions, such as light intensity, temperature, water availability and wind strength, affect plant growth. Plants also modify the environment around them, they release water which cools the air, breakdown the soil to make it suitable for their roots and for other plants and animals and decrease the speed of the wind.
Photosynthesis:-Probably the most important role of plants in the environment is the production of oxygen (O2) and the absorption of carbon dioxide (CO2) from the atmosphere during the process of photosynthesis. Photosynthesis is the basic process for plant life.
We Use Plants in Many Ways
 Plants For Food
Plants For FoodNearly 75% of the the world's food supply is based on seven major crops: wheat, rice, maize (corn), potatoes, barley, cassava and sorghum.
Chocolate is made from the fruit of the cocoa tree.Cocoa beans are roasted, shelled and then crushed. Cocoa butter and cocoa powder are separated. Cocoa powder is then mixed with milk to make chocolate.
Canola- 78% of vegetable oil production is from canola.Canola is pressed from the canola seeds and used as salad oil and frying oilIt is used to make margarine, shortening, baked goods, potato chips and french fries.
Seaweed contains iodine and is used in soup broths and sushi.other products from seaweed include: ice cream, chocolate milk, yogurt, whipped cream, pies, jellies and candies.seaweed products are often used to thicken food (alginate, agar, carrageenan).
Sugar half of the world's sugar comes from sugar beets, located in the sugar beets' roots.roots are shredded, heated in running water and the concentrated clear liquid crystallizes to produce sugar similar to sugar cane.
People use plants for things other than food.
Plants for Fibre
Plants also provide fibre, which is the tissue of plants from the stem, leaves, seeds or roots. Plants provide fibres for clothing, paper and shelter. The aboriginal people from the west coast wove cloth from the bark of the western red cedar tree. Much of our clothing today comes from synthetic (manufactured) material, such as polyester and nylon. Natural fibres also provide resources for cloth:
• Cotton - is a natural fibre that absorbs moisture and then allows it to evaporate easily, making it the world's most important non-edible plant. The cotton fibres come from the plant's seeds. The silky fibres are strong, flexible and have a gradual spiral that causes the strans to intelock when twisted, making them ideal for spinning into thread. The second layer of fibers are shorter and are 'fuzzy' - they are used to make cotton batting, rayon and various types of plastic and paper.
• Hemp - Early makers of jeans used hemp, which is the oldest cultivated fibre plant in the world. Other products included the Bible, sails and ropes. Hemp has a less negative effect on the environment, because it uses less land area than trees, can be harvested in a year, lasts longer than paper, can be recycled up to seven times, chokes out weeds naturally and is not prone to insect pests.
• Flax - is a food and fibre crop. The flax fibres, which are smooth and straight, are taken from the stem of the plant are are two to three times stronger than cotton fibres. Flax fibre is used for making linen paper, linseed oil - which is used as a drying oil in paints and varnish - and in products such as klinoleum and printing inks.
Plants for Medicine
An apple a day keeps the doctor away! Many medicines (over 7000) contain ingredients made from plants. Herbal remedies are a common example of how plants are used to prevent illness. Plant medicines include:
• tea (made from ginger root) - is used to soothe an upset stomach
• tea (made from white spruce and hemlock) to prevent scurvy
• white willow bark - is used to ease pain
• kinnikinick (buffalo berry) was used to treat kidney problems
• opium poppy's seed pod - thick milky fluid provides a powerful pain medication - morphine
• codeine is also found in the poppy - it is used in cough medicines
• quinine - which comes from the cinchona tree - is used to prevent malaria.
Plants for Transportation and Construction
Rubber is one of the most important plant products that people use. Natural rubber comes from the Brazilian rubber tree. Synthetic rubber is made from coal and oil by-products - but natural rubber is also an important ingredient. Canoes were carved from trees by Aboriginal people. Lubricants are provided from coconut and castor bean oils. The construction industry in North America uses wood (softwood lumber from British Columbia) as a building material.
Plants for Fuel
Wood or coal (which is a fossil fuel) are used to heat homes. Sugar can be turned into ethanol and wood can provide methanol (wood alcohol). Fuel from plants is economical, but not energy efficient, because a large amount of energy is needed to grow the plants and a lot of the energy is lost when it is converted to fuel.
Saving the Earth
Having now seen how the plant and animal life are tied in this reciprocal relationship it makes us aware of the necessity to take care of the earth for future generations and ourselves. As part of our third grade curriculum on communities, we would be studying the rights and responsibilities of the people and the government in helping to prevent possible problems with the ecology of the earth.
How does pollution affect plants? How Can We Help?
While we are still able to breathe, there are many days when the pollution levels make it difficult. Chemicals and gases in the air that should not be present cause air pollution. Three main environmental problems that affect plants include: Acid Rain, Global Warming, and Ozone Depletion.
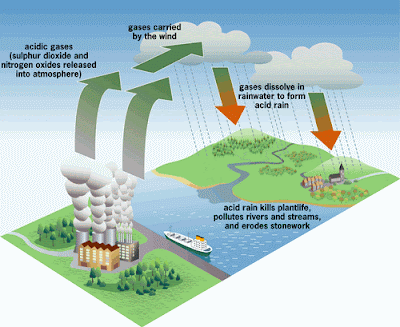
Acid rain begins as normal rain but when it falls through clouds of pollution the rain changes into a weak acid. Water vapor sometimes mixes with the exhaust from cars, and factory smoke stacks as it moves over the land. This combination of nitric oxide and sulfur dioxide dissolves in the clouds. This acid can sometimes be as strong as vinegar.This acid is strong enough to damage trees, dissolve marble, and kill fish in ponds and lakes. Small lakes and ponds can be helped temporarily by the addition of baking soda or limestone. The best cure, however, is to control the pollution that is the cause.
Global Warming or the greenhouse affect is connected to the fact that the earth is a closed ecosystem. The temperatures are kept to a certain level so that plant life can thrive. The earth’s temperature is partly the result of the stratosphere that traps gases in the troposphere and stratosphere. Too much heat would make the earth warmer and with hotter temperatures the balance of life would begin to erode. The so-called greenhouse gases ñ carbon dioxide, water vapor, ozone, methane, nitrous oxide, and chlorofluorocarbons - trap heat that is reflected off the earth’s surface. Nature tries to balance itself, but the things that people do upset it. When people burn wood, coal, and oil, more carbon dioxide is released into the air. The carbon dioxide slows down the movement of heat and acts as an insulator. The result is what would happen if the earth were put inside a closed jar.The earth gets increasingly warm from the sun but it cannot cool off. Trees are an important solution to the problem since the trees help to remove carbon out of the air. Saving the rain forests from destruction would help. Some scientists speculate that if the earth’s atmosphere rises a few degrees it could have a devastating affect on the planet. Plant life would be negatively impacted and the polar ice caps would begin to melt. People often confuse the issue of global warming with that of the ozone depletion but they are two separate occurrences though both are major environmental problems.
The ozone is a thin layer of gas that is about 12 miles up in the earth’s stratosphere. This gas helps to protect the earth by filtering the sunlight that hits the earth. Without that layer higher than normal levels of the sun’s ultraviolet radiation would come down and affect the population. These harmful rays mean more skin cancer, cataracts, and lung problems from increased smog levels. Chemicals like the chlorofluorocarbons in refrigerators and air conditioners harm the ozone layer as they rise into the air. Again people can help if they change their buying habits. Don’t purchase foam products that can damage the ozone when they are burned or put in landfills. Use recyclable paper or plastic cups instead of Styrofoam that is also toxic. Refrigerators and freezers should be kept in good running order and if possible limit use of air conditioning or use a fan instead. Scientists measure Ozone depletion by using satellites. The layer is getting thinner, and over places like Antarctica it is completely gone.
We must make sure that our living resources survive and thrive,in order to have them in the future. As we need plants for our survival, they also need care from us.
...................................................................